Wave Life Sciences shares positive results from their Phase 1b/2a SELECT-HD trial.
Wave announced that their SELECT-HD trial which aimed to lower the expanded Huntingtin gene had seen positive results in the next phase of their trial. Over the course of the 28-week trial the mutant huntingtin gene was lowered by 44% while the healthy gene was preserved.
We are very proud to have demonstrated mHTT (mutated gene) lowering of 46%, with preservation of wtHTT (healthy gene), and are encouraged to see these reductions in mHTT significantly correlating with a slowing in caudate atrophy after just 28 weeks. These results represent a significant achievement for Wave, for the oligonucleotide field, and most importantly, for the Huntington's community.
WVE-003
WVE-003 (the drug administrated in the SELECT-HD trial) was developed with the aim of lowering the mutated huntingtin gene (mHTT) whilst preserving what they call the wild-type huntingtin gene (wtHTT).
The health huntingtin gene (wtHTT protein) supports the health and function of neurons. The mutated huntingtin gene (mHTT) also has a detrimental effect on healthy gene at the protein level, which further decreases the healthy genes function. Only selective lowering of mutant gene has the potential to relieve its negative impact on healthy protein function.
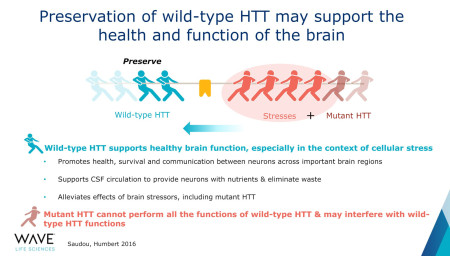
Wild-type huntingtin plays such a critical role in the central nervous system, and it’s very exciting to finally have an opportunity to evaluate mHTT lowering in the context of allele-selectivity and to see positive signals emerging.
SELECT-HD trial
Wave's SELECT-HD drug is called WVE-003. WVE-003 has been developed as a disease-modifying therapeutic for Huntington's disease. Participants received either 30 mg of WVE-003 or a placebo every eight weeks via the spinal fluid followed by 12 weeks of follow-up.
- WVE-003 was generally safe and well-tolerated, with no Serious Adverse Events (SAEs) reported; ventricular volume was in line with natural history
- Significant mHTT (mutant huntingtin) protein lowering was observed throughout the 28-week assessment period
- At 24 weeks (8 weeks after last dose), mean mHTT (mutant huntingtin) lowering in cerebrospinal fluid (CSF) was 46% versus placebo
- At 28 weeks (12 weeks after last dose), mean mHTT (mutant huntingtin) lowering in CSF was 44% versus placebo which supports quarterly or less frequent dosing
- wtHTT (health huntingtin) protein was preserved throughout the 28-week assessment period, validating the selective silencing of the mutant gene. Additionally, statistically significant increases were observed in wtHTT (healthy huntingtin) protein versus placebo
- At 24 weeks (the last MRI assessment), mHTT (mutant huntingtin) reduction was correlated with the slowing of the decline of Total Motor Score (TMS)
We believe these strong data compel a case for accelerated approval for WVE-003, which we plan to discuss with regulators. We would like to express our immense gratitude to the Huntington's community, the study participants, their families, and study site staff for their trust, support, and engagement that have helped us reach this important milestone.




